Multiple Choice
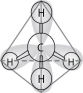 Figure 2.8
Figure 2.8
-In the methane molecule shown in Figure 2.8, bonds have formed that include both the s orbital valence electrons of the hydrogen atoms and the p orbital valence electrons of the carbon. The electron orbitals in these bonds are said to be
A) double orbitals.
B) tetrahedral orbitals.
C) complex orbitals.
D) hybrid orbitals.
E) polar orbitals.
Correct Answer:

Verified
Correct Answer:
Verified
Q36: You have two beakers. One contains a
Q54: Which of the following is true for
Q55: Which one of the atoms shown would
Q56: The atomic number of sulfur is 16.
Q57: About 25 of the 92 natural elements
Q59: Which of the following correctly describes chemical
Q60: One of the buffers that contributes to
Q61: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6147/.jpg" alt=" Figure 2.5 -Figure
Q80: Van der Waals interactions result when<br>A) hybrid
Q130: How many electron pairs are shared between