Multiple Choice
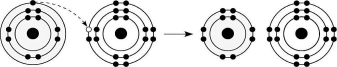 Figure 2.6
Figure 2.6
-What results from the chemical reaction illustrated in Figure 2.6?
A) a cation with a net charge of +1
B) a cation with a net charge of -1
C) an anion with a net charge of +1
D) an anion with a net charge of -1
E) a cation with a net charge of +1 and an anion with a net charge of -1
Correct Answer:

Verified
Correct Answer:
Verified
Q24: Which of the following solutions would require
Q42: Many mammals control their body temperature by
Q52: An atom with atomic number 12 would
Q78: Nitrogen (N) is much more electronegative than
Q79: Glucose has a molecular mass of 180
Q80: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6148/.jpg" alt=" Figure 2.6 -
Q84: Molybdenum has an atomic number of 42.
Q85: How many glucose molecules are contained in
Q87: Which of the following are considered compounds?<br>A)
Q131: The chemical behavior of an atom depends