Multiple Choice
Tooth enamel is composed of the mineral hydroxyapatite. It is essentially insoluble in water with Ksp 2.3 1059, but it reacts with weak acids in the mouth as described by one of the reaction equations below. You can determine the equilibrium constant, K, for this reaction from Ksp and Kw (the equilibrium constant for water autoionization) . How do you do this? 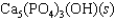
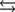
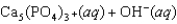
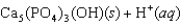
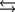
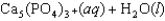
A) K KwKsp
B) K Kw /Ksp
C) K Ksp /Kw
D) K Kw Ksp
E) K Kw Ksp
Correct Answer:

Verified
Correct Answer:
Verified
Q43: Which element may contribute to sudden infant
Q44: Radioactive <sup>90</sup>Sr can substitute for _ in
Q45: Fluoride is added to toothpastes and drinking
Q46: Why are some elements in the human
Q47: Pacemakers implanted in the chests of patients
Q49: The concentration of an ultratrace essential element
Q50: Hearing aid batteries utilize a zinc/air electrochemical
Q51: Enzymes in plants convert nitrate into ammonia.
Q52: Plants convert nitrogen into ammonia. This biological
Q53: Draw two possible Lewis structures of the