Multiple Choice
Enzymes in plants convert nitrate into ammonia. The unbalanced half-reaction equation is given below. What is the stoichiometric coefficient of the nitrite ion in the balanced reaction equation? 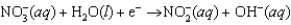
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer:

Verified
Correct Answer:
Verified
Q46: Why are some elements in the human
Q47: Pacemakers implanted in the chests of patients
Q48: Tooth enamel is composed of the mineral
Q49: The concentration of an ultratrace essential element
Q50: Hearing aid batteries utilize a zinc/air electrochemical
Q52: Plants convert nitrogen into ammonia. This biological
Q53: Draw two possible Lewis structures of the
Q54: Hearing aid batteries utilize a zinc/air electrochemical
Q55: The isotope <sup>18</sup>F is used in a
Q56: The ion found in chlorophyll is _<br>A)Zn<sup>2</sup><font