Multiple Choice
Different concentrations of ions on either side of a cell membrane constitute a concentration cell, which is described by the Nernst equation given below. A cell must expend energy to maintain this concentration gradient by moving ions from one side of the membrane to the other. How much energy must be expended to transport 1 mole of sodium ions across a membrane when the sodium ion concentration is 0.10 M on one side of the membrane and 0.010 M on the other side, and the temperature is 37C (body temperature) ? (Given: 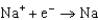 ,
, 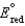 2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K) )
2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K) )
E E - 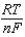 InQ
InQ
A) 50 kJ
B) 260 kJ
C) 6.0 kJ
D) 250 kJ
E) 6,000 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q86: Enzymes in plants convert nitrate into ammonia.
Q87: Plants react urea with water to produce
Q88: A patient is injected with a 5.0
Q89: Enzymes in plants convert nitrate into ammonia.
Q90: Rhenium-186 has medical applications. It decays by
Q91: Selenium is an ultratrace element that is
Q92: Hearing aid batteries utilize a zinc/air electrochemical
Q93: Tooth enamel is composed of the calcium
Q94: Cell membranes assemble _<br>A)to orient nonpolar fatty
Q95: Which metal, by either mass or number