Short Answer
Different concentrations of ions on either side of a cell membrane constitute a concentration cell, which is described by the Nernst equation given below. A cell must expend energy to maintain this concentration gradient by moving ions from one side of the membrane to the other. How much energy must be expended to transport 1 mole of sodium ions across a membrane when the sodium ion concentration is 0.10 M on one side of the membrane and 0.010 M on the other side, and the temperature is 37C (body temperature)?
(Given: 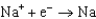 ,
, 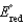 2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K))
2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K))
E E - 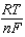 InQ
InQ
Correct Answer:

Verified
Correct Answer:
Verified
Q72: Nitrogen is a major essential element in
Q73: Adding fluoride ions to toothpaste and drinking
Q74: Cell membranes are formed by _<br>A)a lipid
Q75: Calcium carbonate is used to form exoskeletons.
Q76: Hydroxyapatite, which is a component of tooth
Q78: Which of the following elements, by mass,
Q79: The nucleus of a cell has a
Q80: Radioactive tritium, <sup>3</sup>H, is used as a
Q81: Which element, by the number of atoms,
Q82: Calcium is a major essential element in