Short Answer
Hydroxyapatite, which is a component of tooth enamel, is very insoluble in water. For the following reaction, Ksp 2.3 1059. 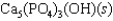
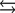
 What is the molar concentration of Ca5(PO4)3 when the pH of the mouth is 4.0?
What is the molar concentration of Ca5(PO4)3 when the pH of the mouth is 4.0?
Correct Answer:

Verified
2.3 F1F1F1...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q71: Which statement describing the mechanisms by which
Q72: Nitrogen is a major essential element in
Q73: Adding fluoride ions to toothpaste and drinking
Q74: Cell membranes are formed by _<br>A)a lipid
Q75: Calcium carbonate is used to form exoskeletons.
Q77: Different concentrations of ions on either side
Q78: Which of the following elements, by mass,
Q79: The nucleus of a cell has a
Q80: Radioactive tritium, <sup>3</sup>H, is used as a
Q81: Which element, by the number of atoms,