Multiple Choice
For the following reaction, which statement AD is not correct? 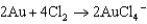
A) Au is the reducing agent.
B) Cl2 is the oxidizing agent.
C) Au is oxidized.
D) The equation is balanced.
E) More than one statement is not correct.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q136: Which statement regarding voltaic cells is not
Q137: The following reaction occurs in a new
Q138: Reduction refers to _<br>A)a decrease in oxidation
Q139: An electrochemical cell is constructed with a
Q140: Consider an electrochemical cell with a Zn
Q142: The oxidation of hydrogen by oxygen is
Q143: Which statement about corrosion is not correct?<br>A)Corrosion
Q144: What kind of chemical reaction occurs at
Q145: Glancing at a periodic table, where do
Q146: Silver tarnishes due to the formation of