Multiple Choice
The oxidation of hydrogen by oxygen is one of the most-used reactions in fuel-cell technology. The overall reaction, which is given below, has a G value of 474 kJ/mol. What is the standard cell potential for this fuel cell? 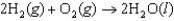
A) 2.46 V
B) 4.91 V
C) 1.23 V
D) 3.05 V
E) 1.50 V
Correct Answer:

Verified
Correct Answer:
Verified
Q137: The following reaction occurs in a new
Q138: Reduction refers to _<br>A)a decrease in oxidation
Q139: An electrochemical cell is constructed with a
Q140: Consider an electrochemical cell with a Zn
Q141: For the following reaction, which statement A<font
Q143: Which statement about corrosion is not correct?<br>A)Corrosion
Q144: What kind of chemical reaction occurs at
Q145: Glancing at a periodic table, where do
Q146: Silver tarnishes due to the formation of
Q147: When a voltaic cell reaches equilibrium, _<br>A)