Multiple Choice
Consider the following chemical reaction used to construct a voltaic cell 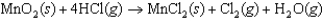 Which one of the following statements is correct?
Which one of the following statements is correct?
A) MnCl2 is produced at the anode.
B) Cl ions are reduced at the cathode.
C) MnO2 is the reducing agent.
D) Three moles of electrons are transferred in this reaction.
E) Electrons travel from Cl to Mn4.
Correct Answer:

Verified
Correct Answer:
Verified
Q67: What is true when a battery (voltaic
Q68: Does pH have an effect on the
Q69: If 15 g of aluminum from an
Q70: A typical D battery has a capacity
Q71: Zinc-air batteries are actively being researched because
Q73: A pH meter uses an electrode arrangement
Q74: An electrochemical cell with a standard hydrogen
Q75: A typical 1.5 V AAA battery has
Q76: The diagram below represents a voltaic cell.
Q77: Lead is a toxic metal. The U.S.