Multiple Choice
Does pH have an effect on the cell potential (Ecell) for the following oxidation-reduction reaction? 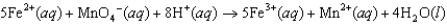
A) Yes, because Ecell values for all redox reactions depend on the pH.
B) Yes, because Ecell values for redox reactions involving the hydronium ion depend on the pH.
C) No, because Ecell values for redox reactions depend only on the major species in the reaction, in this case, Fe2, MnO4, Fe3, and Mn2.
D) No, because Ecell values for redox reactions depend on concentrations and temperatures but not on pH.
E) No, because Ecell values for redox reactions do not depend on the pH.
Correct Answer:

Verified
Correct Answer:
Verified
Q63: If the free-energy change of the following
Q64: Which statement about a voltaic cell is
Q65: How many kilograms of aluminum metal can
Q66: Which cell diagram is correct for the
Q67: What is true when a battery (voltaic
Q69: If 15 g of aluminum from an
Q70: A typical D battery has a capacity
Q71: Zinc-air batteries are actively being researched because
Q72: Consider the following chemical reaction used to
Q73: A pH meter uses an electrode arrangement