Multiple Choice
This is a true story; can you explain what happened? Prior to a really important dinner party, the hostess discovered that her silverware was very tarnished. She needed a quick fix. She remembered reading that the tarnish (Ag2S) would be removed if you immersed the silverware in a hot solution of baking soda (NaHCO3) in a pan lined with aluminum foil. So she did, and so it was, but she noticed a bit of a rotten egg smell (H2S) being produced. Metal/Metal ion 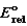 silver/silver(I)
silver/silver(I)
0) 799
Aluminum/aluminum(III) 1.677
A) Aluminum ions react with S2, form an aluminum sulfide precipitate, and gaseous carbon dioxide is released.
B) Silver ions in the presence of the baking soda, (NaHCO3) , oxidize the sulfide to elemental sulfur that attacks the aluminum foil, which produces the smelly aluminum sulfide.
C) The aluminum acts as a reducing agent for the silver(I) in the silver sulfide; then the bicarbonate ion protonates the sulfide ion that is released.
D) Aluminum is plated onto the silver surface, making it shiny again, and then the reaction of the bicarbonate with the aluminum oxide releases CO2.
E) Silver in Ag2S reduces the aluminum, becomes metallic silver in the process, and releases hydrogen sulfide, H2S.
Correct Answer:

Verified
Correct Answer:
Verified
Q127: The capacity of a battery usually is
Q128: Oxidation refers to _<br>A)an increase in oxidation
Q129: The oxidation of hydrogen by oxygen is
Q130: How long would it take to electroplate
Q131: The numerical value of the Faraday constant
Q133: The average electrical current delivered if 1.0
Q134: What is the change in free energy
Q135: An electrochemical cell is represented below. Which
Q136: Which statement regarding voltaic cells is not
Q137: The following reaction occurs in a new