Multiple Choice
The oxidation of hydrogen by oxygen is one of the most-used reactions in fuel-cell technology. The overall reaction, which is given below, has a G value of 237 kJ/mol. What is the standard cell potential for this fuel cell? 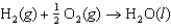
A) 2.46 V
B) 4.91 V
C) 1.23 V
D) 3.05 V
E) 1.50 V
Correct Answer:

Verified
Correct Answer:
Verified
Q124: A typical AA battery has a capacity
Q125: If the free-energy change of the following
Q126: Use the table of standard reduction potentials
Q127: The capacity of a battery usually is
Q128: Oxidation refers to _<br>A)an increase in oxidation
Q130: How long would it take to electroplate
Q131: The numerical value of the Faraday constant
Q132: This is a true story; can you
Q133: The average electrical current delivered if 1.0
Q134: What is the change in free energy