Multiple Choice
Use the table of standard reduction potentials below to identify the metal or metal ion that is the weakest oxidizing agent. Standard Reduction
Potentials (volts) in Aqueous Solution 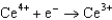 1.70
1.70 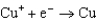 0.520
0.520 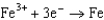 0.036
0.036 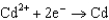 0.400
0.400 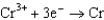 0.730
0.730 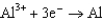 1.66
1.66
A) Cu
B) Cr3
C) Cd2
D) Ce3
E) Al
Correct Answer:

Verified
Correct Answer:
Verified
Q35: Consider the following standard reduction potentials. Reduction
Q36: Calculate the value of K for the
Q37: If the free-energy change of a voltaic
Q38: The work involved in moving exactly 1
Q39: A typical hearing aid battery has a
Q41: Because of recent advances in recovery technology,
Q42: For the following reaction, predict the pH
Q43: Which one of the following items does
Q44: The diagram below represents a voltaic cell.
Q45: For the following reaction, predict the pH