Multiple Choice
For the following reaction, predict the pH if the pressure of O2 is 3.00 atm and the bromide ion concentration is 2.10 M. The measured cell potential (Ecell) was 0.100 V. 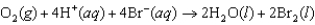 Half reactions:
Half reactions: 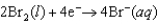 E 1.077 V
E 1.077 V 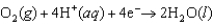 E 1.229 V
E 1.229 V
A) 4.31
B) 3.17
C) 2.59
D) 1.32
E) 0.66
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q37: If the free-energy change of a voltaic
Q38: The work involved in moving exactly 1
Q39: A typical hearing aid battery has a
Q40: Use the table of standard reduction potentials
Q41: Because of recent advances in recovery technology,
Q43: Which one of the following items does
Q44: The diagram below represents a voltaic cell.
Q45: For the following reaction, predict the pH
Q46: In the reaction below, _ is the
Q47: A voltaic cell is constructed based on