Multiple Choice
Consider the voltaic cell based on the following reaction: 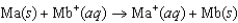 Two students are assigned to measure the standard cell potential. Yvonne claims that both solutions of ions have to be at exactly 1.00 M concentration, but Zelda is sure that the measurement will be the same if the concentrations of the two solutions are equal, but not necessarily 1.00 M. What do you think? (The temperature is controlled at 25C, so that's not an issue.)
Two students are assigned to measure the standard cell potential. Yvonne claims that both solutions of ions have to be at exactly 1.00 M concentration, but Zelda is sure that the measurement will be the same if the concentrations of the two solutions are equal, but not necessarily 1.00 M. What do you think? (The temperature is controlled at 25C, so that's not an issue.)
A) Yvonne is right because by definition standard cell potentials must be measured at concentrations of 1.00 M.
B) Yvonne is right because she understands the Nernst equation and what it describes.
C) Zelda is right because she understands the Nernst equation and what it describes.
D) Zelda is right because cell potentials do not depend on the concentration.
E) Both are right because cell potentials do not depend on the concentration.
Correct Answer:

Verified
Correct Answer:
Verified
Q157: The capacity of a battery usually is
Q158: The diagram below represents a voltaic cell.
Q159: Chromium often is electroplated on other metals
Q160: An electrochemical cell is constructed with a
Q161: The thermite reaction, shown below, is very
Q162: The magnitude of the charge on a
Q164: The following reaction occurs in basic solution.
Q165: Chromium often is electroplated on other metals
Q166: For a chemical reaction to be considered
Q167: Using the following data, determine the standard