Multiple Choice
Using the following data, determine the standard cell potential E for an electrochemical cell with zinc as the anode, lead as the cathode, and solutions of the respective ions. 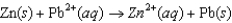 Half-reaction Standard Reduction Potential
Half-reaction Standard Reduction Potential 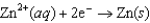 0.763
0.763 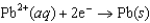 0.126
0.126
A) (1.274 V)
B) (0.637 V)
C) (0.889 V)
D) (0.889 V)
E) (0.637 V)
Correct Answer:

Verified
Correct Answer:
Verified
Q157: The capacity of a battery usually is
Q158: The diagram below represents a voltaic cell.
Q159: Chromium often is electroplated on other metals
Q160: An electrochemical cell is constructed with a
Q161: The thermite reaction, shown below, is very
Q162: The magnitude of the charge on a
Q163: Consider the voltaic cell based on the
Q164: The following reaction occurs in basic solution.
Q165: Chromium often is electroplated on other metals
Q166: For a chemical reaction to be considered