Short Answer
Using the following data, determine the standard cell potential 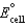 for the electrochemical cell constructed using the following reaction, where zinc is the anode and lead is the cathode.
for the electrochemical cell constructed using the following reaction, where zinc is the anode and lead is the cathode. 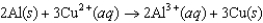 Half-reaction Standard Reduction Potential (V)
Half-reaction Standard Reduction Potential (V) 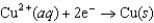 0.34
0.34 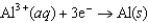 1.66
1.66
Correct Answer:

Verified
F...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q115: The electrodes on batteries are labeled <font
Q116: From the following table of standard reduction
Q117: Which cell diagram is correct for this
Q118: What is the mass of copper used
Q119: A NiMH battery uses _ as the
Q121: Which of the following is not an
Q122: What is the cell potential for this
Q123: A NiMH battery uses _ as the
Q124: A typical AA battery has a capacity
Q125: If the free-energy change of the following