Multiple Choice
If the free-energy change of the following voltaic cell 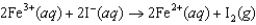 is 46.3 kJ, what is the standard potential of the cell?
is 46.3 kJ, what is the standard potential of the cell?
A) (0.080 V)
B) (0.080 V)
C) (0.240 V)
D) (0.240 V)
E) (0.480 V)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q120: Using the following data, determine the standard
Q121: Which of the following is not an
Q122: What is the cell potential for this
Q123: A NiMH battery uses _ as the
Q124: A typical AA battery has a capacity
Q126: Use the table of standard reduction potentials
Q127: The capacity of a battery usually is
Q128: Oxidation refers to _<br>A)an increase in oxidation
Q129: The oxidation of hydrogen by oxygen is
Q130: How long would it take to electroplate