Short Answer
If the free-energy change of the following voltaic cell 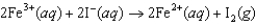 is 46.3 kJ, what is the standard potential of the cell?
is 46.3 kJ, what is the standard potential of the cell?
Correct Answer:

Verified
F...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
F...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q77: Lead is a toxic metal. The U.S.
Q78: An electrochemical cell has both a silver
Q79: The diagram below represents a voltaic cell.
Q80: Because of recent advances in recovery technology,
Q81: Which statement about this concentration cell is
Q83: Which statement regarding battery-powered electric cars is
Q84: Copper metal is purified by electrolysis. How
Q85: The energy supplied by a battery can
Q86: A voltaic cell is constructed based on
Q87: Use the table of standard reduction potentials