Multiple Choice
Use the table of standard reduction potentials below to identify the metal or metal ion that is the weakest reducing agent. Standard Reduction
Potentials (volts) in Aqueous Solution 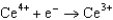 1.70
1.70 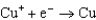 0.520
0.520  0.036
0.036 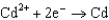 0.400
0.400 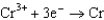 0.73
0.73 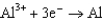 1.66
1.66
A) Cr
B) Fe
C) Cu
D) Ce4
E) Al3
Correct Answer:

Verified
Correct Answer:
Verified
Q82: If the free-energy change of the following
Q83: Which statement regarding battery-powered electric cars is
Q84: Copper metal is purified by electrolysis. How
Q85: The energy supplied by a battery can
Q86: A voltaic cell is constructed based on
Q88: Consider the following standard reduction potentials. Reduction
Q89: The diagram below represents a voltaic cell.
Q90: Sodium carbonate is produced using the Solvay
Q91: Which one of the following is not
Q92: What must be true about the standard