Multiple Choice
Given the following data relevant to the combustion of ethanol, determine the free energy of formation for liquid ethanol, C2H5OH. 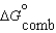 (C2H5OH, l) 930.7 kJ/mol
(C2H5OH, l) 930.7 kJ/mol 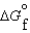 (CO2, g) 394.4 kJ/mol
(CO2, g) 394.4 kJ/mol 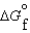 (H2O, g) 105.6 kJ/mol
(H2O, g) 105.6 kJ/mol
A) (1,640.3 kJ/mol)
B) (244.2 kJ/mol)
C) (174.9 kJ/mol)
D) (174.9 kJ/mol)
E) (244.2 kJ/mol)
Correct Answer:

Verified
Correct Answer:
Verified
Q80: What types of motion does a molecule
Q81: Which of the following statements is/are correct?
Q82: Which of the processes A-D will lead
Q83: One of the following statements A-D may
Q84: Which of the relationships between the free-energy
Q86: Give an example of a spontaneous and
Q87: The dissolution of ammonium nitrate in water
Q88: If for a given chemical reaction at
Q89: Draw a graph of entropy versus temperature
Q90: Which of the following figures illustrates best