Multiple Choice
Consider substances that exist as liquids under standard state conditions. What must be the relationship between the enthalpy and free energy of formation for the liquid and the gaseous form of such a substance? (Read as more negative and as less negative.)
A) 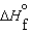 (l )
(l ) 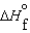 (g) and
(g) and 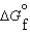 (l )
(l ) 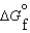 (g)
(g)
B) 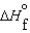 (l )
(l ) 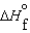 (g) and
(g) and 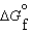 (l )
(l ) 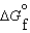 (g)
(g)
C) 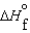 (l )
(l ) 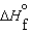 (g) and
(g) and 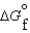 (l )
(l ) 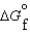 (g)
(g)
D) 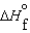 (l )
(l ) 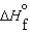 (g) and
(g) and 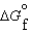 (l )
(l ) 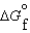 (g)
(g)
E) No strict relationship between these values applies.
Correct Answer:

Verified
Correct Answer:
Verified
Q113: What is the entropy change if 4.500
Q114: For a particular hypothetical reaction, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"
Q115: Which of the following must be true
Q116: Which of the following graphs best depicts
Q117: Which of the following will have the
Q119: When a solution of DNA in water
Q120: Which of the following must be true
Q121: When plotting ln K vs. 1/T, a
Q122: Which of the following must be true
Q123: Of the three modes of molecular motion-vibration,