Multiple Choice
For the following exothermic reaction, predict under which conditions the reaction will be spontaneous. 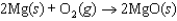
A) The reaction is always spontaneous.
B) The reaction is never spontaneous.
C) The reaction is spontaneous at high temperatures.
D) The reaction is spontaneous at low temperatures.
E) Insufficient data is provided to answer this question.
Correct Answer:

Verified
Correct Answer:
Verified
Q132: Noxious NO gas can form from N<sub>2</sub>
Q133: What is the standard entropy change when
Q134: When you increase the volume of a
Q135: The symbol <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"
Q136: The standard molar entropy of lead(II) bromide
Q138: If for a given chemical reaction at
Q139: If a reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="If a
Q140: Values for <font face="symbol"></font>H<font face="symbol"></font> and <font
Q141: Which of the following statements is/are correct?
Q142: Boltzmann derived the relationship S <font face="symbol"></font>