Multiple Choice
If a reaction 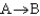 has
has 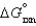 0, then when the reaction is at equilibrium there will be ________
0, then when the reaction is at equilibrium there will be ________
A) absolutely no B that is formed.
B) smaller quantities of B and larger quantities of A.
C) equal quantities of A and B.
D) large quantities of B and smaller quantities of A.
E) absolutely no A remaining.
Correct Answer:

Verified
Correct Answer:
Verified
Q134: When you increase the volume of a
Q135: The symbol <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"
Q136: The standard molar entropy of lead(II) bromide
Q137: For the following exothermic reaction, predict under
Q138: If for a given chemical reaction at
Q140: Values for <font face="symbol"></font>H<font face="symbol"></font> and <font
Q141: Which of the following statements is/are correct?
Q142: Boltzmann derived the relationship S <font face="symbol"></font>
Q143: What is the value of the equilibrium
Q144: Determine the value of <font face="symbol"></font>G<font face="symbol"></font>