Multiple Choice
Alcohols for use as biofuels can be produced from glucose that is obtained from starch and cellulose in plants. Use the information in the table below to determine the temperature range, if any, at which this reaction is spontaneous. 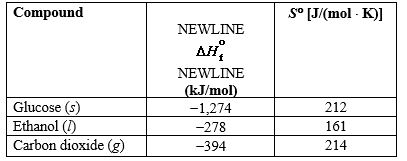
A) Reaction is spontaneous at high temperatures.
B) Reaction is spontaneous at low temperatures.
C) Reaction is spontaneous at all temperatures.
D) Reaction is not spontaneous at any temperature.
E) Whether the reaction is spontaneous cannot be predicted from the information provided.
Correct Answer:

Verified
Correct Answer:
Verified
Q43: For a chemical reaction with a positive
Q44: A reaction with a low enthalpy of
Q45: Carbon atoms can be found in a
Q46: At body temperature, many proteins have a
Q47: If <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="If
Q49: During a spontaneous chemical reaction, it is
Q50: The standard molar entropy of magnesium fluoride
Q51: Determine the value of <font face="symbol"></font>G<font face="symbol"></font>
Q52: The enthalpy of vaporization for toluene (C<sub>7</sub>H<sub>8</sub>)
Q53: Determine the standard entropy of N<sub>2</sub>(g) given