Multiple Choice
If 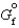 for a reaction is 13.4 kJ/mol, what is K for the reaction at 2C?
for a reaction is 13.4 kJ/mol, what is K for the reaction at 2C?
A) 3.51 102
B) 2.85 103
C) 1.00
D) 2.23
E) 4.47 101
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q42: What is the entropy change to the
Q43: For a chemical reaction with a positive
Q44: A reaction with a low enthalpy of
Q45: Carbon atoms can be found in a
Q46: At body temperature, many proteins have a
Q48: Alcohols for use as biofuels can be
Q49: During a spontaneous chemical reaction, it is
Q50: The standard molar entropy of magnesium fluoride
Q51: Determine the value of <font face="symbol"></font>G<font face="symbol"></font>
Q52: The enthalpy of vaporization for toluene (C<sub>7</sub>H<sub>8</sub>)