Multiple Choice
Given the following two measurements of the equilibrium constant for a reaction, calculate H for the reaction. 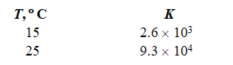
A) 255 kJ/mol
B) (271 kJ/mol)
C) 3,250 kJ/mol
D) (3,250 kJ/mol)
E) (271 kJ/mol)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: A sketch of the free energy for
Q16: A reaction is not spontaneous at any
Q17: Care must be taken when dissolving solid
Q18: In an experiment, 1.00 atm of N<sub>2</sub>(g)
Q19: Because the triple point of water is
Q21: The standard entropy of diamond is 2.4
Q22: The equilibrium constant for a reaction is
Q23: In an experiment, 1.00 atm of N<sub>2</sub>(g)
Q24: Rank the following compounds from smallest to
Q25: <font face="symbol"></font>S<sub>sys</sub> can be directly related to