Multiple Choice
A sketch of the free energy for a hypothetical chemical equilibrium is shown here. What part of the plot on the axis representing the relative quantities of reactants and products corresponds to a value of Q that is less than K? 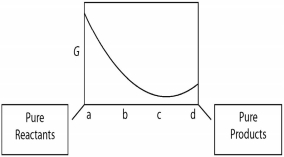
A) a to b
B) b to c
C) a to c
D) b to d
E) c to d
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Before class, students were seated at three
Q11: Which statement about the reaction below, where
Q12: Indicate which one of the following reactions
Q13: Which of the following is in the
Q14: The symbol <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"
Q16: A reaction is not spontaneous at any
Q17: Care must be taken when dissolving solid
Q18: In an experiment, 1.00 atm of N<sub>2</sub>(g)
Q19: Because the triple point of water is
Q20: Given the following two measurements of the