Short Answer
Given the following data, determine the molar free energy of combustion for propane gas, C3H8. 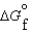 (C3H8, g)23.5 kJ/mol
(C3H8, g)23.5 kJ/mol 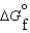 (CO2, g)394.4 kJ/mol
(CO2, g)394.4 kJ/mol 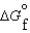 (H2O, g)105.6 kJ/mol
(H2O, g)105.6 kJ/mol
Correct Answer:

Verified
F...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q63: Perfect crystals of carbon monoxide (CO) are
Q64: Which, if any, of statements A-D is
Q65: If for a given chemical reaction at
Q66: The values of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="The values
Q67: Which statement is true of the ideal
Q69: Suppose <font face="symbol"></font>G<font face="symbol"></font> is 25.0 kJ/mol
Q70: If K for a reaction is large,
Q71: Hydrochloric acid (HCl) reacts with sodium hydroxide
Q72: What type of motion is depicted in
Q73: Alcohols for use as biofuels can be