Multiple Choice
At what point in the following titration curve for a weak acid being titrated with a strong base is the pH equal to the pKa of the acid? The x-axis scale goes from 0.0 mL to 20.0 mL. The sharp rise is at 10.0 mL. 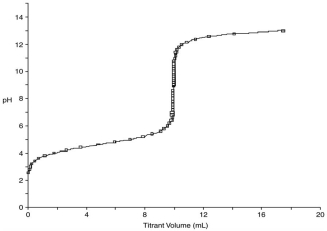
A) 0.0 mL
B) 5.0 mL
C) 9.0 mL
D) 10.0 mL
E) 18.0 mL
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Calculate the K<sub>sp</sub> for Li<sub>3</sub>PO<sub>4 </sub>if at
Q14: Determine the molar concentration of Cl<font face="symbol"><sup></sup></font>
Q15: In a titration of monoprotic acids and
Q16: Which combination of solutions is the best
Q17: Lead poisoning used to be treated by
Q19: A ligand is any _ forming a
Q20: A phosphate buffer solution (25.00 mL sample)
Q21: The bonds between the zinc ion and
Q22: To simulate the pH of blood, which
Q23: Given the following titration curves, provide a