Essay
Given the following titration curves, provide a potential reaction that describes each titration.
a) 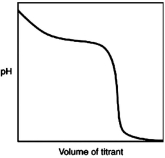
b) 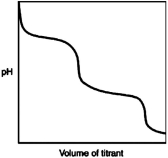
Correct Answer:

Verified
a)  (weak base-stron...
(weak base-stron...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
a)  (weak base-stron...
(weak base-stron...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q18: At what point in the following titration
Q19: A ligand is any _ forming a
Q20: A phosphate buffer solution (25.00 mL sample)
Q21: The bonds between the zinc ion and
Q22: To simulate the pH of blood, which
Q24: Describe the similarities and differences between a
Q25: The pK<sub>a</sub> of a weak acid was
Q26: Explain how a buffer solution manages to
Q27: Glycolic acid, which is a monoprotic acid
Q28: Phenylephrine (PE, see the structure below) is