Multiple Choice
What type of packing best describes the layering pattern shown below? 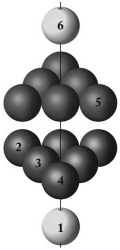
A) body-centered closest-packed
B) hexagonal closest-packed
C) random-packed
D) cubic closest-packed
E) cubic packing
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q84: An approximately spherical allotrope of carbon containing
Q85: Consider the three unit cells below for
Q86: Graphite and diamond are examples of _
Q87: A face-centered cubic unit cell contains _
Q88: Copper crystallizes in a face-centered cubic pattern.
Q90: Nylon is a _<br>A)polyester.<br>B)polyamide.<br>C)polyaminoacid.<br>D)polyaromatic.<br>E)polyether.
Q91: A face-centered cubic unit cell has a(n)
Q92: Ionic solids have _ melting points, and
Q93: In ionic solids, one ion often occupies
Q94: How many nearest neighbor atoms are there