Short Answer
Consider the three unit cells below for ionic solids. In each unit cell, one of the ions is arranged in one of the standard unit cell structures while the other is found between these sites. What are the names of the minerals associated with each of these structures? I ________; II ________;
III ________. 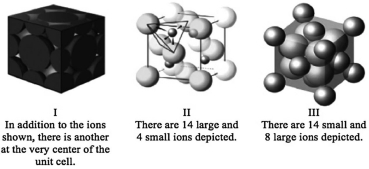
Correct Answer:

Verified
rock salt ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q80: The monomer for polystyrene is shown below.
Q81: The two types of closest-packed lattices are
Q82: Iron (Fe) crystallizes as a body-centered unit
Q83: Which of the following monomers is used
Q84: An approximately spherical allotrope of carbon containing
Q86: Graphite and diamond are examples of _
Q87: A face-centered cubic unit cell contains _
Q88: Copper crystallizes in a face-centered cubic pattern.
Q89: What type of packing best describes the
Q90: Nylon is a _<br>A)polyester.<br>B)polyamide.<br>C)polyaminoacid.<br>D)polyaromatic.<br>E)polyether.