Multiple Choice
Calculate the lattice energy of magnesium chloride from the following data: First ionization energy of Mg: 738 kJ/mol
Second ionization energy of Mg: 1,451 kJ/mol
Electron affinity of Cl: 349 kJ/mol
Energy to vaporize Mg: 147.1 kJ/mol
Cl2 bond energy: 243 kJ/mol
Energy change for the reaction: 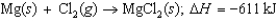
A) 1,246 kJ/mol
B) (779 kJ/mol)
C) (2,492 kJ/mol)
D) 2,492 kJ/mol
E) (1,246 kJ/mol)
Correct Answer:

Verified
Correct Answer:
Verified
Q126: In the following Born-Haber cycle for the
Q127: A newspaper article suggested using a fertilizer
Q128: In the following Born-Haber cycle for the
Q129: A solution is prepared by adding 1.50
Q130: What would be the boiling point of
Q132: Which of the following ionic compounds would
Q133: What would be the freezing point of
Q134: Click, Clack, and Jim have identical icy
Q135: Which one of the ionic compounds below
Q136: A solution of 5.00 g of sodium