Multiple Choice
In the following Born-Haber cycle for the formation of sodium chloride from its elements, which step (A-E) corresponds to the lattice energy for NaCl? 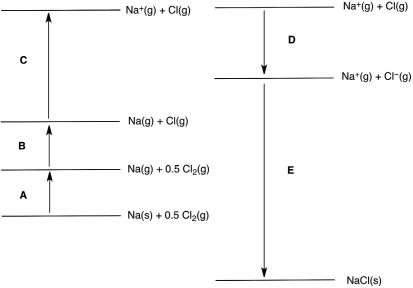
A) A
B) B
C) C
D) D
E) E
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q121: Coulomb's law states that the interaction energy
Q122: Rank the following ionic compounds based on
Q123: A solution is prepared by adding 0.250
Q124: Which graph best describes how the vapor
Q125: What is the molarity of a 0.923
Q127: A newspaper article suggested using a fertilizer
Q128: In the following Born-Haber cycle for the
Q129: A solution is prepared by adding 1.50
Q130: What would be the boiling point of
Q131: Calculate the lattice energy of magnesium chloride