Multiple Choice
Which of the following diagrams best shows a set of polar molecules interacting through dipole-dipole interactions?
A) 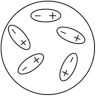
B) 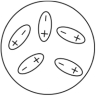
C) 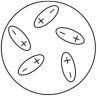
D) 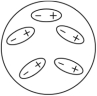
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q112: Which of the substances a-d in the
Q113: The density of water decreases as it
Q114: Henry's law constant (mol/L · atm) for
Q115: Of all the noble gases, _ has
Q116: At the triple point of a substance,
Q118: Which of the following representations best shows
Q119: A hydration sphere forms around an ion
Q120: The solubility of any gas in a
Q121: For a molecule to exhibit dipole-dipole interactions,
Q122: On the phase diagram below, identify the