Multiple Choice
Henry's law constant (mol/L · atm) for oxygen dissolving in blood is 3.74 102 mol/L · atm at body temperature, 37C. Calculate the molar concentration of oxygen in blood for an alpine climber where the atmospheric pressure is 0.45 atm. The mole fraction of oxygen in air is 0.209.
A) 7.8 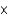 103 M
103 M
B) 3.5 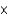 103 M
103 M
C) 2.3 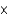 102 M
102 M
D) 1.3 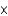 102 M
102 M
E) 0.11 M
Correct Answer:

Verified
Correct Answer:
Verified
Q109: A solute is most likely to be
Q110: Given the van der Waals a constant
Q111: Of all the noble gases, _ has
Q112: Which of the substances a-d in the
Q113: The density of water decreases as it
Q115: Of all the noble gases, _ has
Q116: At the triple point of a substance,
Q117: Which of the following diagrams best shows
Q118: Which of the following representations best shows
Q119: A hydration sphere forms around an ion