Multiple Choice
When potassium bromide dissolves in water, which picture best represents the solution? 
A) 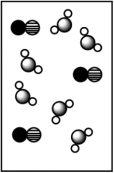
B) 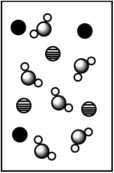
C) 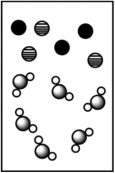
D) 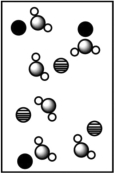
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q58: Which intermolecular force is caused by an
Q59: On the phase diagram below, identify the
Q60: Identify which of the following alkanes has
Q61: The relative energies (strengths) of the intermolecular
Q62: Dipole-dipole interactions typically are not as strong
Q64: Point c in the phase diagram below
Q65: Describe the utility of the Clausius-Clapeyron equation.
Q66: Which of the following compounds would you
Q67: Gasoline is primarily a mixture of hydrocarbons
Q68: Which of the following gases do you