Multiple Choice
On the phase diagram below, identify the normal boiling point. 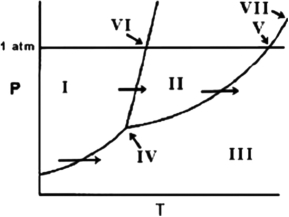
A) VI
B) V
C) VII
D) IV
E) III
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q54: Melting occurs in going from region _
Q55: Which molecule has the largest dipole?<br>A)methane (CH<sub>4</sub>)<br>B)ammonia
Q56: Which of the following nonpolar molecules will
Q57: What does the line indicated by the
Q58: Which intermolecular force is caused by an
Q60: Identify which of the following alkanes has
Q61: The relative energies (strengths) of the intermolecular
Q62: Dipole-dipole interactions typically are not as strong
Q63: When potassium bromide dissolves in water, which
Q64: Point c in the phase diagram below