Multiple Choice
If water contains about 42 mg of oxygen per liter at 20C and 1.0 atm, what would be the value of Henry's law constant for oxygen dissolving in water? The mole fraction of oxygen in air is 0.21.
A) 0.25 mol/(L atm)
B) 1.3 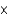 103 mol/(L atm)
103 mol/(L atm)
C) 1.9 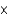 102 mol/(L atm)
102 mol/(L atm)
D) 6.3 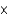 10-3 mol/(L atm)
10-3 mol/(L atm)
E) 0.010 mol/(L atm)
Correct Answer:

Verified
Correct Answer:
Verified
Q154: Which of the following compounds would you
Q155: Henry's law constant (mol/L · atm) for
Q156: Condensation occurs in going from region _
Q157: The phase diagram for carbon dioxide is
Q158: Which of the following compounds do you
Q160: Indicate which of the following pairs of
Q161: Which of the following gases do you
Q162: Air (consisting mostly of nitrogen (N<sub>2</sub>), oxygen
Q163: The solubility of a compound is determined
Q164: Explain why the rate of evaporation of