Multiple Choice
The phase diagram for carbon dioxide is shown below. What are the phase changes in order as carbon dioxide is heated from -90oC to 50oC, at 1 atm pressure? 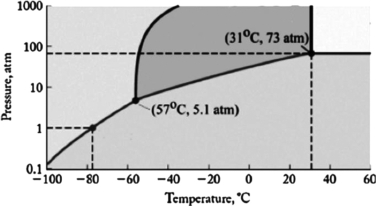
A) solid  gas
gas
B) solid  liquid
liquid  gas
gas
C) liquid  gas
gas
D) gas  liquid
liquid  solid
solid
E) gas  liquid
liquid
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q152: Which of the following substances has a
Q153: Which is the dominant interaction that leads
Q154: Which of the following compounds would you
Q155: Henry's law constant (mol/L · atm) for
Q156: Condensation occurs in going from region _
Q158: Which of the following compounds do you
Q159: If water contains about 42 mg of
Q160: Indicate which of the following pairs of
Q161: Which of the following gases do you
Q162: Air (consisting mostly of nitrogen (N<sub>2</sub>), oxygen