Multiple Choice
What does the line indicated by the arrow in the following phase diagram represent? 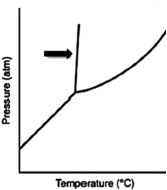
A) solid-liquid boundary
B) solid-gas boundary
C) liquid-gas boundary
D) triple point
E) solid-solid boundary
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q121: For a molecule to exhibit dipole-dipole interactions,
Q122: On the phase diagram below, identify the
Q123: What structural characteristics must a molecule have
Q124: Which of the following solvents will involve
Q125: Which of the following statements does not
Q127: The aroma from almonds and cherries is
Q128: Viscosity is a measure of a substance's
Q129: For each of the following pairs of
Q130: Which of the following molecules will have
Q131: At ambient temperature, F<sub>2</sub> and Cl<sub>2 </sub>are