Multiple Choice
What is the valence electron molecular orbital electron configuration of O2?
A) 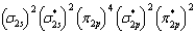
B) 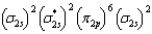
C) 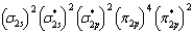
D) 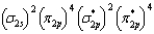
E) 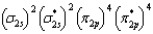
Correct Answer:

Verified
Correct Answer:
Verified
Q97: Which statement regarding the electronic structure of
Q98: Consider the molecule below-how many sp<sup>2</sup> hybridized
Q99: The bond angle in a trigonal planar
Q100: What is the valence electron molecular orbital
Q101: Determine the molecular geometry of CF<sub>2</sub>Cl<sub>2</sub>.<br>A)linear<br>B)bent<br>C)trigonal bipyramidal<br>D)tetrahedral<br>E)trigonal
Q103: Identify the hybridization of the atomic orbitals
Q104: All homonuclear diatomic molecules _<br>A)have polar bonds.<br>B)are
Q105: Electrical and thermal conductivity in metals _<br>A)is
Q106: Which one of the following molecules is
Q107: A triatomic molecule with a bond angle