Multiple Choice
Which one of the following molecules is paramagnetic? These molecules are described by the MO energy diagram below. These molecular orbitals are formed from the 2s and 2p atomic orbitals. 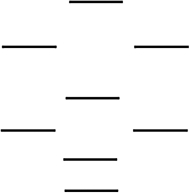
A) Li2
B) C22
C) N22
D) N22
E) C2
Correct Answer:

Verified
Correct Answer:
Verified
Q101: Determine the molecular geometry of CF<sub>2</sub>Cl<sub>2</sub>.<br>A)linear<br>B)bent<br>C)trigonal bipyramidal<br>D)tetrahedral<br>E)trigonal
Q102: What is the valence electron molecular orbital
Q103: Identify the hybridization of the atomic orbitals
Q104: All homonuclear diatomic molecules _<br>A)have polar bonds.<br>B)are
Q105: Electrical and thermal conductivity in metals _<br>A)is
Q107: A triatomic molecule with a bond angle
Q108: Identify the molecular geometry of the molecular
Q109: Which statement about <font face="symbol"></font>and <font face="symbol"></font>bonds
Q110: For the series methane, ammonia, and water,
Q111: Which of the following molecules has a