Essay
Describe the valence bond picture of bonding in ethylene, which is shown below. Identify the number of valence electrons, the number of bonds, the number of bonds, and the hybridization of the carbon atomic orbitals. 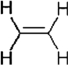
Correct Answer:

Verified
Ethylene has 12 valence electr...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q178: Carbonyl dihalides (COX<sub>2</sub> with X = I,
Q179: Predict the bond order of F<sub>2</sub><font face="symbol"><sup></sup></font>.<br>A)0<br>B)0.5<br>C)1<br>D)1.5<br>E)2
Q180: Predict the bond order of the NO<font
Q181: Predict the bond angle indicated in SF<sub>4</sub>.
Q182: Draw a structure showing the geometry of
Q183: Which type of molecular orbital is used
Q184: Which bond is the most polar?<br>A)H-C<br>B)H-N<br>C)H-O<br>D)H-Cl<br>E)H-F
Q185: Identify the electron pair and molecular geometries
Q187: Which of the following compounds has the
Q188: Identify the hybridization of the three carbon