Multiple Choice
Identify the hybridization of the three carbon atoms labeled in the following molecule. 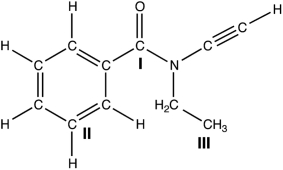 I II III I II III
I II III I II III
A) sp3, sp3, sp2
B) sp3, sp3, sp3
C) sp3, sp2, sp3
D) sp2, sp2, sp3
E) sp3, sp2, sp2
Correct Answer:

Verified
Correct Answer:
Verified
Q178: Carbonyl dihalides (COX<sub>2</sub> with X = I,
Q179: Predict the bond order of F<sub>2</sub><font face="symbol"><sup></sup></font>.<br>A)0<br>B)0.5<br>C)1<br>D)1.5<br>E)2
Q180: Predict the bond order of the NO<font
Q181: Predict the bond angle indicated in SF<sub>4</sub>.
Q182: Draw a structure showing the geometry of
Q183: Which type of molecular orbital is used
Q184: Which bond is the most polar?<br>A)H-C<br>B)H-N<br>C)H-O<br>D)H-Cl<br>E)H-F
Q185: Identify the electron pair and molecular geometries
Q186: Describe the valence bond picture of bonding
Q187: Which of the following compounds has the