Multiple Choice
What is the formal charge of each atom (from left to right) in the following resonance structure of SN2? 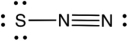
A) 0, 0, 0
B) (1, 1, 0)
C) (2, 1, 0)
D) (2, 1, 1)
E) (1, 1, 1)
Correct Answer:

Verified
Correct Answer:
Verified
Q35: Which molecule has the longest single bond?<br>A)H<sub>2</sub><br>B)HF<br>C)HCl<br>D)HBr<br>E)HI
Q36: Draw the Lewis structure of OCS.
Q37: Which of the following is an example
Q38: Which of the following atoms can have
Q39: Which structure for dinitrogen sulfide (N<font face="symbol"></font>N<font
Q41: Which of the following is the correct
Q42: Draw the Lewis structure for the nitrate
Q43: Sodium nitrite (NaNO<sub>2</sub>) is often used in
Q44: Which of the following bonds is primarily
Q45: Triple bonds_<br>A)involve sharing of 3 total electrons