Multiple Choice
What is the formal charge of each atom (from left to right) , neglecting the hydrogens, in the following resonance structure of CH2N2? 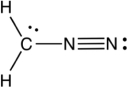
A) 0, 0, 0
B) (1, 1, 0)
C) 0, 1, 1
D) 0, 1, 1
E) (1, 1, 1)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Which of the following is the shortest
Q2: Which statement A-D about chemical bonds is
Q3: The following Lewis symbol corresponds to which
Q4: Which of these statements correctly describes why
Q6: O-S-S-O has three resonance Lewis structures that
Q7: Which of the following represents the best
Q8: Which one of the following molecules violates
Q9: The formal charge of an atom in
Q10: Assign the formal charges to each atom
Q11: Indicate which of the following molecules has