Essay
Assign the formal charges to each atom in the resonance structure of ozone (O3) shown below. 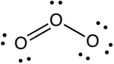
Correct Answer:

Verified
 The formal charges ...
The formal charges ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q5: What is the formal charge of each
Q6: O-S-S-O has three resonance Lewis structures that
Q7: Which of the following represents the best
Q8: Which one of the following molecules violates
Q9: The formal charge of an atom in
Q11: Indicate which of the following molecules has
Q12: Which of these statements correctly describes the
Q13: Which statement about the most stable Lewis
Q14: How many valence electrons are there in
Q15: To absorb infrared radiation, a molecule must